This poster was presented as part of the British Paediatric Neurology Association 2023 conference, at the Royal College of Physicians in Edinburgh. The poster presents the education resource co-created by Azafaros and Cognitant Group Limited for the GM1 and GM2 patient community about the natural history study, PRONTO.
Abstract
Recruiting participants to rare disease natural history studies is inherently challenging due to the small patient population to recruit from. This challenge is often further compounded as motivation to engage in paediatric trials is reduced by the lack of clear and understandable information materials shared with caregivers about what will be expected from them, and their child, throughout participation. Effective recruitment materials also play a pivotal role in ensuring consent is fully informed from the outset and subsequently reducing attrition as the trial commences.
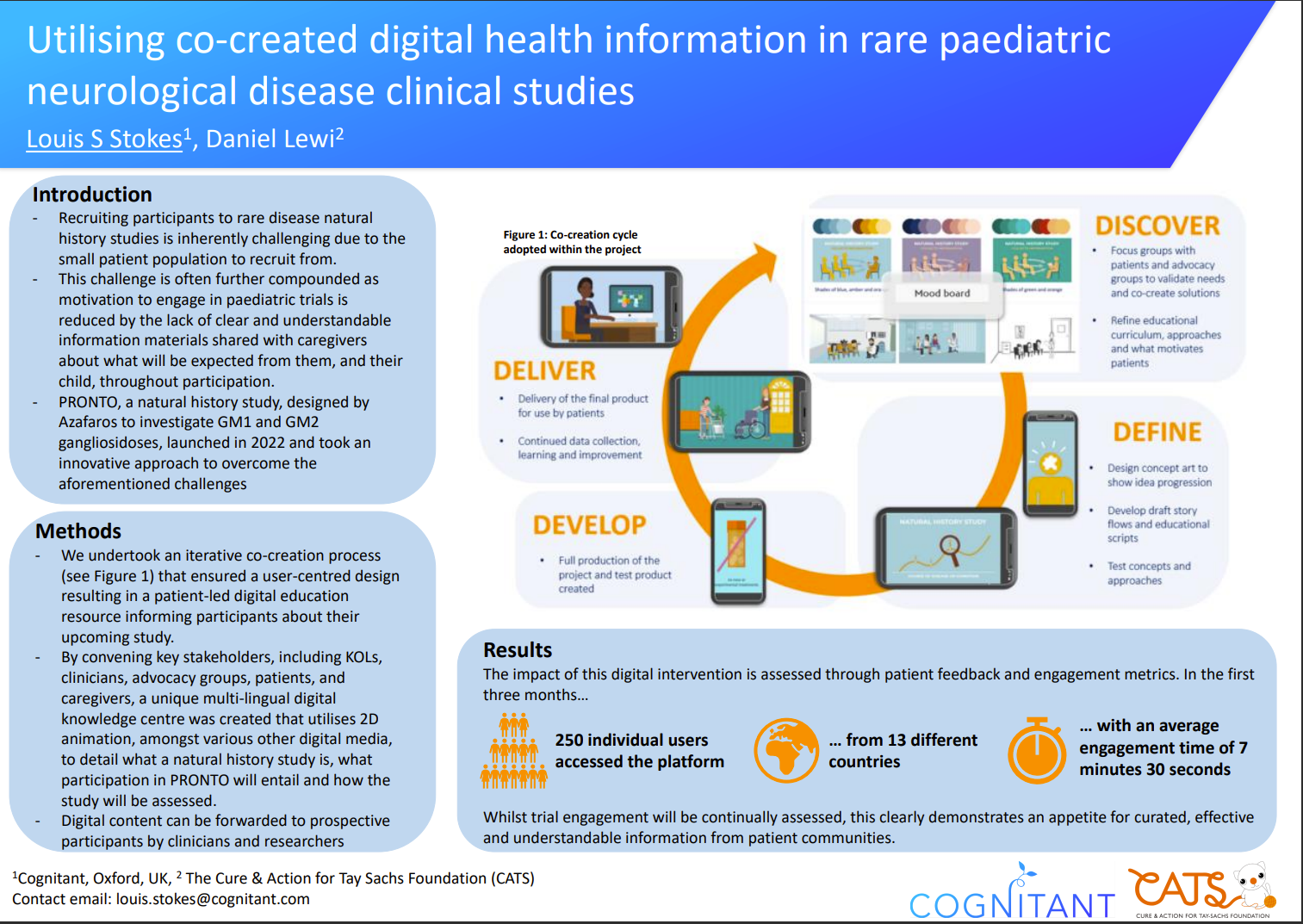
PRONTO, a natural history study, designed by Azafaros to investigate GM1 and GM2 gangliosidoses, launched in 2022 and took an innovative approach to overcome the aforementioned challenges. In partnership with Cognitant, specialists in creating health education resources, they undertook an iterative co-creation process that ensured a user-centred design resulting in a patient-led digital education resource informing participants about their upcoming study.
By convening key stakeholders, including KOLs, clinicians, advocacy groups, patients, and caregivers, from Europe and the US, Azafaros created a unique multi-lingual digital knowledge centre that utilises 2D animation, amongst various other digital media, to detail what a natural history study is, what participation in PRONTO will entail and how the study will be assessed. By hosting content digitally, clinicians and advocacy groups can send information directly to prospective participants and their caregivers, enabling them to view and share key trial information away from the clinic, and in their own time.
The impact of this digital intervention is assessed through patient feedback and engagement metrics. In the first 3 months over 250 individual users from 13 different countries have accessed the knowledge centre, with an average engagement time of 7 minutes and 30 seconds. Whilst trial engagement will be continually assessed, this clearly demonstrates an appetite for curated, effective and understandable information from patient communities.
Cognitant
Looking to empower people with health information for better patient outcomes?
Related News
Kidney Research UK invests in Healthinote patient education platform which could support >15m people with long term health conditions
April, 2025
Oxford, January 2025 – Kidney Research UK, the leading charity dedicated to kidney health, has made a significant investment in Cognitant Group Ltd, a leading...
Webinar Insights – Compliance vs Patient Engagement: Can They Co-Exist?
April, 2025
Is compliance really a barrier to meaningful patient engagement in pharma, or are outdated myths holding us back? In this recent webinar, Dr Tim Ringrose,...
Funding awarded to innovations that support early diagnosis and rehabilitation of Stroke patients
March, 2025
SBRI Healthcare, an Accelerated Access Collaborative (AAC) initiative, in partnership with the Health Innovation Network, has awarded £2.5 million for the development of five innovations...